India rejected J&J’s patent on TB drug
Why in news :
- Recently, the Indian Patent Office rejected an application by pharmaceutical giant Johnson & Johnson (J&J) to extend its patent on the drug bedaquiline beyond July 2023.
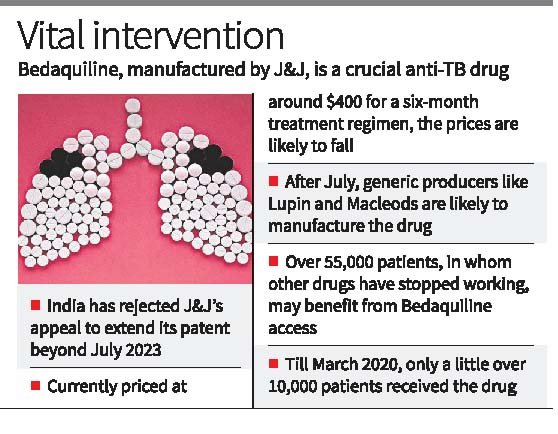
- This opens the door for drug manufacturers to produce generic versions of bedaquiline, which are expected to be more affordable and to contribute to India’s goal of eliminating TB by 2025.
- Bedaquiline is a drug in tablet form used to treat drug-resistant tuberculosis (TB).
About TB and its treatment :
- Tuberculosis(TB) is an infectious disease usually caused by Mycobacterium tuberculosis (MTB) bacteria.
- Tuberculosis generally affects the lungs, but it can also affect other parts of the body.
- Most infections show no symptoms, in which case it is known as latent tuberculosis.
- Around 10% of latent infections progress to active disease which, if left untreated, kill about half of those affected.
- Typical symptoms of active TB are chronic cough with blood-containingmucus, fever, night sweats, and weight loss.
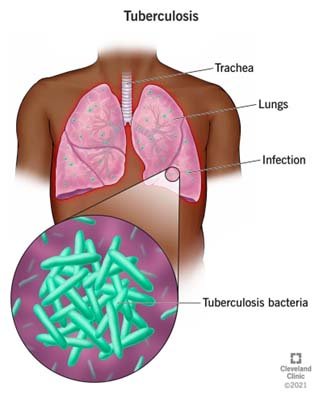
- Tuberculosis is spread from one person to the next through the air when people who have active TB in their lungs cough, spit, speak, or sneeze.
- People with Latent TB do not spread the disease.
What is drug-resistant TB?
- As of 2017, India accounted for around one-fourth of the world’s burden of multi-drug-resistant (MDR) TB and of extensively-drug-resistant (XDR) TB.
- MDR TB resists treatment by at least two frontline drugs in TB treatment, isoniazid and rifampicin.
- XDR TB resists these two drugs as well as fluoroquinolones and any second-line injectable drug.
- It can be treated by strictly adhering to the doses and frequencies of drugs prescribed by a physician.
- Deviations from this schedule can lead the bacteria to become drug-resistant.
- Drug-resistant TB is harder to treat.
- One important option for those diagnosed with pulmonary MDR TB is bedaquiline.
- In 2018, the World Health Organization replaced two injectable drugs for MDR TB with an oral regimen that included bedaquiline.
Why was the patent application rejected?
- J&J’s patent application was for a fumarate salt of a compound to produce bedaquiline tablets.
- According to the Indian Patent Act 1970 Section 2(1)(ja), an ‘inventive step’ is an invention that is “not obvious to a person skilled in the art”.
- The Patent Office rejected the application on these and other grounds, including Sections 3d and 3e of the Act.
Syllabus : Prelims + Mains; GS III – Patents


